Systems neuroscientists are pushing aside their electrophysiology rigs to make room for the tools of 'optogenetics'. Lizzie Buchen reports from a field in the process of reinvention.

Sabes's training was in physics, machine learning and human perception, and his lab has been working with humans and non-human primates to develop models of how the brain turns perceptions into actions; for example, seeing a fly and swatting it away. He's not alone in his molecular-biology naivety at the Keck Center — there is no cell-culture facility, no PCR machine and no bench-top centrifuge. The centre's one ice machine spits out large cubes instead of the crushed ice routinely used for chilling reagents — it was ordered by mistake, and no one has cared enough to fix the situation. Sabes and his colleagues have had no need for such apparatus. Researchers in their field of 'systems neuroscience' try to understand how networks of neurons process sensations and control behaviours such as learning and decision-making. And up to this point, much of their progress has been made using electrophysiology, stimulating and recording from the brains of animals as they perform a task or develop a new skill.
Now though, advances in a five-year-old field called optogenetics are convincing these scientists to crack open molecular-biology textbooks. Using a hybrid of genetics, virology and optics, the techniques involved enable researchers to instantaneously activate or silence specific groups of neurons within circuits with a precision that electrophysiology and other standard methods do not allow. Systems neuroscientists have longed for such an advance, which allows them their first real opportunity to pick apart the labyrinthine jumble of cell types in a circuit and test what each one does. "It has revolutionized my approach to science," says Antonello Bonci, a neurophysiologist at the UCSF Ernest Gallo Clinic and Research Center in Emeryville who began using the technique in 2007. "It can clarify unequivocally the role of specific classes of cells, and solve controversies that have been going on for many, many years." Among the clarifications sought is the precise function of 'place' cells, hippocampal neurons that fire only when an animal finds itself in a specific location; another is the function of complex activity patterns observed when an animal is paying attention or executing a movement.
“Optogentics can solve controversies that have been going on for many, many years.”
A field's evolution
The transition phase isn't easy. Optogenetic tools were first used in cell cultures and mice, which are amenable to genetic manipulation. Now systems neuroscientists must adapt them to function in organisms they traditionally study such as rats, birds and primates. With the technical challenge comes a personal one, as researchers leave their experimental comfort zone for a new field.Most, however, anticipate that any discomfort will be worthwhile. "This is God's gift to neurophysiologists," says Robert Desimone, director of the McGovern Institute for Brain Research at the Massachusetts Institute of Technology (MIT) in Cambridge, who has been using electrophysiology for more than 30 years. "Molecular techniques were always beyond us, so this is our first opportunity. It's a revolution. But we're catching up to the revolution that had been going on for the rest of the world."
Optogenetics started out in 2005, when a team at Stanford University in California led by Karl Deisseroth and his then-postdoc Ed Boyden inserted a light-sensitive channel from green algae, called channelrhodopsin-2 (ChR2), into neurons growing in a dish. Exposed to a pulse of blue light, the channels opened and a flood of positive ions poured into the neurons, making them fire1. Within a year, 30 labs had contacted Deisseroth to ask for the technology. By March 2010, Deisseroth had sent protocols or genetic constructs to more than 500 labs around the world, and Boyden, now at MIT, had sent them to in excess of 250.
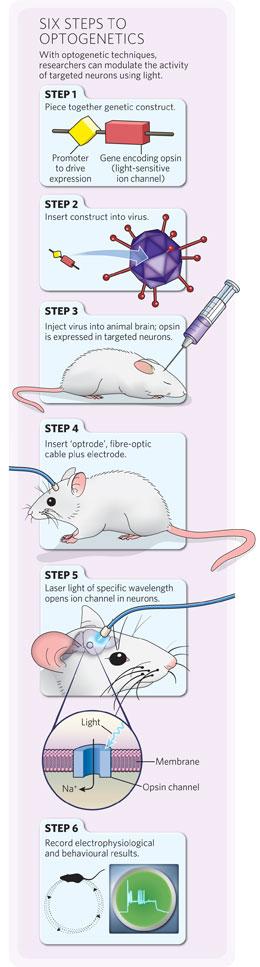
Click for a larger version.
The technique has been refined greatly in the past five years, but the basic steps are the same (see 'Six steps to optogenetics'). First, researchers create a genetic construct containing the ChR2 gene or another 'opsin' gene, along with genetic elements that control its expression — for example, a specific 'promoter' sequence. Then, they package up the construct in a virus. When the virus is injected into an animal's brain, it widely infects neurons and delivers the construct, but the opsin is expressed in only a subgroup of cells with the necessary machinery to activate its promoter. The opsin proteins sit in the membrane of those neurons, and researchers trigger them with light of a specific wavelength, typically delivered through an optic fibre threaded through an animal's skull. Deisseroth and Boyden have discovered or engineered many other opsins that allow neurons to be manipulated in different ways, including neural silencers.The technique is popular, but complex. The fastest adopters were those who work with cells grown in vitro and animals such as flies, worms and mice, for which they could take advantage of established genetic tools and well-characterized animal lines. "The number of tangible results are still of the order of half a dozen or so. But we're on the rising phase of the exponential," says Karel Svoboda, a neuroscientist at the Howard Hughes Medical Institute's Janelia Farm research campus in Ashburn, Virginia, who last year started mapping mouse cortical circuits using optogenetics.
From the start, these developments sent quivers through the systems-neuroscience community. "When I first saw this stuff, at a meeting where Karl was presenting some of the earliest results, I thought, 'Oh God, finally'," says Loren Frank, another neuroscientist at the Keck Center. "And then I thought, oh great. Here I was, I'd gotten pretty good at the stuff I thought I needed to do, and now I have to learn an entirely new field."
 Frank — who says he has "always had a bit of molecular-biology envy" — began collaborating with Deisseroth to use optogenetics on rats just over three years ago, for his studies of place cells and hippocampal circuits. Silencing or activating specific cells could help show whether or how they help an animal explore a new location or recognize a familiar one. "If you want to actually understand the system, you have to start trying to get control of the system and actually dissect the circuit," says Frank. Standard techniques have not allowed that type of dissection. Electrodes inserted into an animal's brain typically stimulate hundreds of thousands of cells; using lesions or drugs also hits circuits like a hammer. Optogenetics could be a scalpel, turning particular neurons in a circuit on or off within milliseconds.
Frank — who says he has "always had a bit of molecular-biology envy" — began collaborating with Deisseroth to use optogenetics on rats just over three years ago, for his studies of place cells and hippocampal circuits. Silencing or activating specific cells could help show whether or how they help an animal explore a new location or recognize a familiar one. "If you want to actually understand the system, you have to start trying to get control of the system and actually dissect the circuit," says Frank. Standard techniques have not allowed that type of dissection. Electrodes inserted into an animal's brain typically stimulate hundreds of thousands of cells; using lesions or drugs also hits circuits like a hammer. Optogenetics could be a scalpel, turning particular neurons in a circuit on or off within milliseconds. From brain to behaviour
By April 2009, Frank was making progress in rats, achieving ChR2 expression in a discrete set of hippocampal neurons and getting them to fire. And down the hall, Sabes was ready to try the technique in primates as part of his efforts to understand circuits involved in hand-eye coordination. "I knew optogenetics was on the horizon, and it was potentially exciting, but realistically, I just didn't know anything about this stuff," he says.Sabes and two songbird researchers at the Keck Center, Michael Brainard and Allison Doupe, teamed up with Frank, Deisseroth and another colleague, Linda Wilbrecht, a neuroscientist at the Gallo Center. The group, one of the first big collaborations aiming to apply optogenetics to rats, birds and primates, received a US$1.6-million National Institutes of Health grant in September 2009 through the financial stimulus. The neuroscientists describe what they'd like to do — in Sabes's case, for example, alter patterns of activity that occur in the parietal lobe when an animal reaches for something, and work out which patterns are important for planning, initiating or adjusting the movement. Wilbrecht's lab leads efforts to generate the appropriate constructs and select the best viruses, and Deisseroth tries to build new viral and optogenetic tools that they need.
One huge advantage for mouse researchers has been the ready-made bank of animal lines engineered to express an enzyme called Cre recombinase in subsets of neurons, such as all dopaminergic ones, which they can use in combination with specially designed genetic constructs to achieve the cellular specificity they want. In other animals, a promoter must be found that will only be turned on in dopaminergic neurons. Some promoters and viruses that work in mice do not work in rats or primates, meaning that researchers have to start from scratch. Progress has been faster in rats because the animals are relatively easy to breed and are similar enough to mice for methods to be transferable. In March 2009, Deisseroth was the first to publish a rat optogenetic study, in which he manipulated circuit components in a rat model of Parkinson's disease to work out which parts might account for the relief afforded by deep-brain stimulation3.
An additional complication for primate researchers is that primate brains are larger than those of rodents, making it difficult to ensure the injected virus and the activating light penetrate deep enough. And troubleshooting in monkeys will take much longer than in rats, given the long lifetime and high value — experimental, financial and ethical — of the animals. But in April 2009, Desimone and his colleagues worked with Boyden to publish the first experiment showing that viruses can be used to insert opsin channels and control neural activity in a macaque.
“It felt like garage-development days, cutting stuff up to see what works.”
In January, with the optrode inserted into the region where the virus had been injected two months earlier, Sabes flipped on a blue laser and watched a screen showing electrical readout from the optrode. Yellow waveforms flashed across the screen, paired with a flurry of clicking noises: spikes of neural activity.
Experts on hand
Like Sabes, the few other electrophysiologists starting to tackle primate optogenetics are keeping experts in the technique close by to avoid making a novice's mistakes (see 'Opto school'). "It's critical that at least the initial phase is collaborative," says Krishna Shenoy, a primate electrophysiologist at Stanford University who has been working closely with Deisseroth for two years to explore how neurons in the brain's motor cortex control arm movements.
Boyden's paper is still the only optogenetics publication on primates, and the technique is some way from generating new discoveries. It's still not known whether the opsins will be expressed consistently for the year or two required to train monkeys in sophisticated behaviours and then record from neurons repeatedly. "If it only expresses at the right level for a few months and then dies off or expresses too much, it's just untenable," says Shenoy.All these questions take time and money to answer, and not every lab has an appetite for the work. "It's probably going to have a big impact, and I'm definitely interested," says Tirin Moore, a neurobiologist at Stanford. "But I'm less interested in pioneering the approach in monkeys and more interested in using the tools once it's clear that they work."
Meanwhile, Boyden and Deisseroth are hammering out some of the problems. Last month, Deisseroth reported a system in mice that could make detailed knowledge of promoters unnecessary: two viruses containing separate genetic constructs are injected into two connected brain regions. Only neurons that run between the two regions will receive doses of both constructs, which then interact to express the opsin5. Boyden is designing a light source that would weigh a fraction of a gram so that animals can walk around freely rather than being tethered to a fibre-optic cable.
"Soon enough, this is going to be standard technology," says Sabes. "The hardware will be there, the viruses will be there, there will be a handful of constructs that everyone agrees works reasonably well." That aim is likely to be furthered by a two-year, $14.9-million grant from the US Defense Advanced Research Projects Agency in Arlington, Virginia, that Sabes, Deisseroth and six other labs, led by Shenoy, won in April. The team will attempt to use primate optogenetics to explore brain repair after injury, including possible light-based neural prosthetics, devices that might stimulate appropriate patterns of activity in the surviving neurons.
For now, Sabes is still trying to analyse the results of his first monkey experiment. He has sent the animal's brain to a friend competent in histology, who will slice it, paper thin, onto slides. Then Sabes will have to grapple with a fluorescent microscope for the first time, trying to work out where and how well the opsin was actually expressed.
"I'm sure it's not that hard," says Sabes, "but I've never done it".
Lizzie Buchen is a freelance writer based in San Francisco.
No comments:
Post a Comment